Introduction:
Patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL), even when treated with novel targeted agents, generally have only limited benefit (Rule et al., Leukemia 2018). The triple combination of the first-generation Bruton tyrosine kinase inhibitor (BTKi), ibrutinib, the BCL-2 inhibitor, venetoclax, and anti-CD20 monoclonal antibody, obinutuzumab has demonstrated preliminary safety and efficacy in R/R and treatment naïve (TN) MCL (Le Gouill et al., Blood 2021). We therefore hypothesized that given the favorable toxicity profile of the second-generation BTKi, acalabrutinib, when given in combination with venetoclax and obinutuzumab (AVO), would be effective and well tolerated in both R/R and TN MCL. Here, we report the results of the phase I dose-finding cohort of AVO in patients with R/R MCL.
Methods:
This is an investigator-initiated, multicenter, multi-cohort phase I/II trial (NCT04855695). The primary endpoints of the phase I portion are the recommended phase II dose (RP2D) and the safety and tolerability of AVO in R/R MCL. The study was designed to evaluate up to 2 dose levels of acalabrutinib, starting with the FDA-approved dose (100 mg po bid, dose level 0 [DL0]) followed by a lower dose (100 mg po daily, DL-1) if dose limiting toxicities (DLTs) occurred in > 1/6 patients in DL0 during the first 4 cycles. Patients were treated in 28-day cycles with acalabrutinib starting with cycle 1, followed by obinutuzumab in cycle 2 (100 mg IV on day 1, 900 mg IV on day 2, 1000 mg IV on days 8 and 15, and on day 1 of cycles 3-7), and venetoclax (weekly dose ramp-up starting in cycle 3 to a target dose of 400 mg daily). Acalabrutinib and venetoclax were continued indefinitely and obinutuzumab maintenance was given every 2 cycles starting with cycle 9 for 12 doses. Patients with progressive disease (PD) or who were unable to receive > 80% of the doses of acalabrutinib and venetoclax during the first 4 cycles were replaced. Key eligibility criteria included R/R MCL after at least one anti-CD20 mAb-based therapy, ECOG PS ≤ 2, and adequate hematologic and organ function. Key exclusion criteria were progression or relapse following prior BTKi or BCL-2 inhibitor and CNS involvement. CTCAE v5 and Lugano criteria were used to evaluate toxicity and efficacy, respectively.
Results:
As of July 26, 2023, 9 patients in DL0 were evaluable for response as 3 patients were replaced prior to reaching the DLT window. The median age was 67 (range 63-79). 78% were male. Median number of prior treatments was 1 (range 1-2). 33% relapsed after autologous stem cell transplant and 22% had primary refractory disease. All patients had advanced stage MCL, with stage IV disease in 89%. MIPI score was 22% high, 56% intermediate, and 22% low risk. Ki67 index ≥ 30% in 44% and ≥ 50% in 33%. 33% patients had complex karyotype, 22% TP53 mutation, 33% p53 IHC expression > 50%, and 33% blastoid variant.
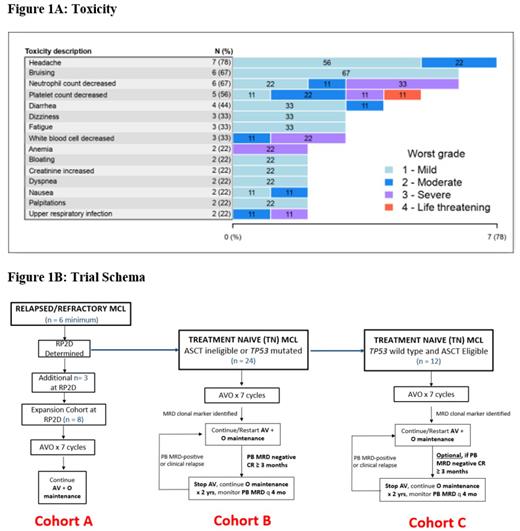
No DLTs were observed and DL0 was determined to be the RP2D. All 9 patients were evaluable for toxicity (Figure 1A). Hematologic toxicity included neutropenia (67%; 33% Gr 3), thrombocytopenia (56%; 22% Gr 3/4), and anemia (22%; all Gr 3). The most common toxicities were headache (77%; all Gr 1/2), bruising (67%; all Gr 1), diarrhea (44%; all Gr 1/2), and upper respiratory infection (22%; 11% Gr 3). There were no serious adverse events. There was no occurrence of febrile neutropenia, atrial fibrillation, bleeding, or laboratory or clinical tumor lysis syndrome.
Median follow-up was 6 months (range 1-21). ORR was 78% (7/9) and CR rate was 67% (6/9) after 3 cycles of AVO. Both patients with TP53 mutation and 2/3 patients with blastoid variant achieved CR. Of the 3 patients replaced prior to the DLT window, 2 patients had PD and 1 patient was unable to receive > 80% of the acalabrutinib and venetoclax doses due to grade 4 thrombocytopenia, although achieved CR. 5 patients (56%) remain on treatment and in CR. 1 patient achieved PR after cycle 3 but progressed after cycle 7. Median overall and progression-free survival were not reached.
Conclusions:
AVO is safe and feasible in patients with R/R MCL with no DLTs observed. RP2D was determined at the full FDA approved dosage of all three drugs. Preliminary efficacy is encouraging in this high-risk R/R MCL population. The phase II TN transplant ineligible or TP53 mutated MCL cohort is now enrolling with minimal residual disease (MRD)-guided time-limited therapy (Figure 1B). The phase I R/R expansion cohort will also open to enrollment.
OffLabel Disclosure:
Armand:ATB Therapeutics: Consultancy; Affimed Therapeutics: Research Funding; Genentech/Roche: Consultancy, Research Funding; Regeneron: Consultancy; Xencor: Consultancy; Adaptive Biotechnologies: Research Funding; IGM: Research Funding; AstraZeneca: Consultancy, Research Funding; MSD: Consultancy, Research Funding; Kite - a Gilead company: Research Funding; ADC Therapeutics: Consultancy; Tessa Therapeutics: Consultancy; Foresight Diagnostics: Consultancy; GenMab: Consultancy; Enterome: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Merck: Consultancy, Honoraria, Research Funding. Merryman:Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Research Funding; Merck: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alphasights: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees. Ahn:AstraZeneca: Consultancy; BeiGene: Consultancy. Crombie:Seagen: Consultancy; Karyopharm: Consultancy; Genmab: Consultancy; ADC therapeutics: Consultancy; Kite: Consultancy; Incyte: Consultancy; Roche: Research Funding; Merck: Research Funding; Abbvie: Research Funding; Bayer: Research Funding. LaCasce:Research to Practice: Consultancy; Seagen, Kite Pharma: Membership on an entity's Board of Directors or advisory committees. Jacobson:Daiichi Sanko: Consultancy, Honoraria; Bluebird Bio: Consultancy, Honoraria; Instil Bio: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Travel support; Bristol Myers Squibb: Consultancy, Honoraria; Axis: Speakers Bureau; Clinical Care Options: Speakers Bureau; Precision BioSciences: Consultancy, Honoraria, Other: Travel support; Nkarta: Consultancy, Honoraria; Humanigen: Consultancy, Honoraria, Other: Travel support; AbbVie: Consultancy, Honoraria; ImmPACT Bio: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Ispen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Other: Travel support; Lonza: Consultancy, Honoraria, Other: Travel support; Kite, A Gilead Company: Consultancy, Honoraria, Research Funding. Jacobsen:Celgene: Research Funding; UpToDate: Patents & Royalties; Bayer: Honoraria; Merck: Honoraria, Research Funding; Hoffman-LaRoche: Research Funding; Daiichi: Honoraria; BMS: Honoraria; Pharmacyclics: Research Funding. Davids:Ascentage Pharma: Consultancy, Research Funding; Adaptive Biosciences: Consultancy; Aptitude Health: Consultancy; BeiGene: Consultancy; BMS: Consultancy; Curio Science: Consultancy; Eli Lilly: Consultancy; Genentech: Consultancy, Research Funding; Janssen: Consultancy; Merck: Consultancy; Mingsight Pharmaceuticals: Consultancy; AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Surface Oncology: Research Funding; Novartis: Research Funding; Takeda: Consultancy; TG Therapeutics: Consultancy, Research Funding; Secura Bio: Consultancy; Research to Practice: Consultancy; MEI Pharma: Research Funding; ONO Pharmaceuticals: Consultancy. Brown:Pfizer: Consultancy; Numab Therapeutics: Consultancy; Acerta/AstraZeneca: Consultancy; MEI Pharma: Research Funding; SecuraBio: Research Funding; Grifols Worldwide Operations: Consultancy; Loxo/Lilly: Consultancy, Research Funding; Kite: Consultancy; iOnctura: Consultancy, Research Funding; Hutchmed: Consultancy; Pharmacyclics: Consultancy; Merck: Consultancy; Abbvie: Consultancy; Genentech/Roche: Consultancy; Gilead: Research Funding; BeiGene: Consultancy, Research Funding; Alloplex Biotherapeutics: Consultancy; TG Therapeutics: Research Funding. Riedell:Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Celgene/ Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Calibr: Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Xencor: Research Funding; CRISPR Therapeutics: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy; Sana Biotechnology: Consultancy; Roche: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; CVS Caremark: Consultancy; MorphoSys: Research Funding; Janssen: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees; Nkarta: Research Funding; Fate Therapeutics: Research Funding; Pharmacyclics: Consultancy. Murakami:Novartis AG: Membership on an entity's Board of Directors or advisory committees; imCORE (Genentech/Roche): Research Funding.
Venetoclax, an oral BCL-2 inhibitor, and Obinutuzumab, an anti-CD20 monoclonal antibody are not FDA approved for treatment of mantle cell lymphoma. Acalabrutinib, a second generation BTK inhibitor, is FDA approved for treatment of relapsed/refractory mantle cell lymphoma. The combination of acalabrutinib, venetoclax, and obinutuzumab is off-label and not approved for treatment of mantle cell lymphoma.